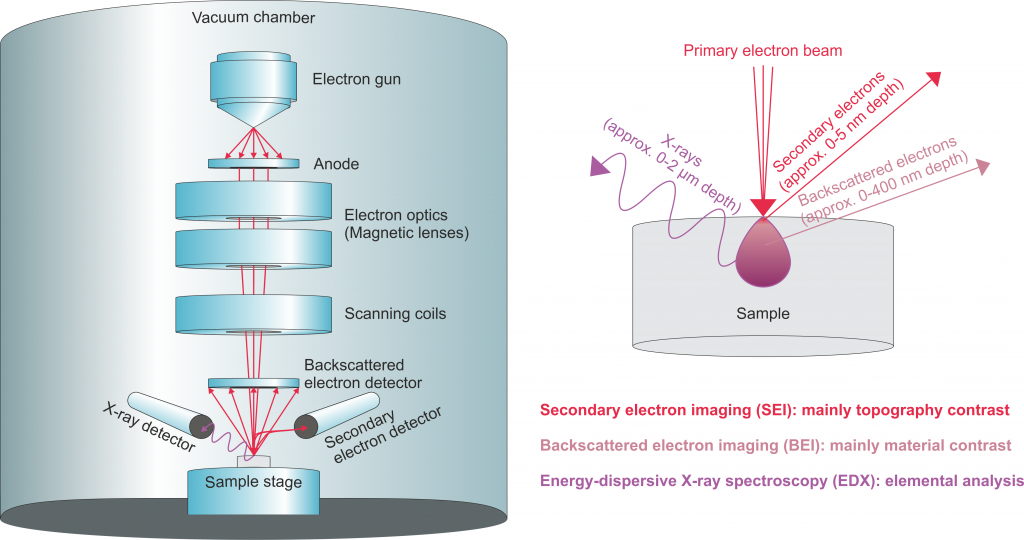
In scanning-electron microscopy (SEM), images are generated by raster-scanning a focused electron beam over a sample and detecting the secondary electrons emitted or the electrons backscattered by the sample. Because electrons can be focused more tightly than light, SEM provides access to spatial resolutions in the nanometer range.
In order to increase the mean free path of electrons, the electron optics and sample are placed into a vacuum chamber. Thus, samples have to be vacuum-stable. Depending on the employed detector, SEM provides different image contrasts, which are based on different penetration depths and sample properties.
Secondary electron imaging (SEI)
In secondary electron imaging (SEI) the secondary electrons emitted from the atoms of the sample are detected. As a part of the excitation energy is consumed for the extraction of electrons from the electron clouds (work function), with their relatively low kinetic energy secondary electrons can only escape from the top few nanometers of the sample to reach the detector. The image contrast mainly reflects the surface topography of the sample.
Backscattered electron imaging (BEI)
The backscattered primary electrons reaching the detector typically represent the top few 100 nm of a sample. Not primarily influenced by surface corrugation, BEI mainly visualizes the distribution of different chemical elements, which is also referred to as material contrast. High-order-number elements scatter the incoming electrons efficiently and therefore appear bright in BE images.
Energy-dispersive X-ray spectroscopy (EDX)
SEM instruments often also provide X-ray emission spectroscopy with an energy-dispersive spectrometer (EDX). Sample atoms that absorb incoming electrons can emit X-ray spectra containing element-specific emission lines. Typically, X-rays generated within the top 2 µm of a sample can reach the detector. Either single spectra from specific spots or EDX maps representing spatial distributions of chemical elements can be acquired.

 ORCID 0000-0001-9708-931X
ORCID 0000-0001-9708-931X